The BRAIN Foundation
Synchrony 2025 Highlights
Held on August 22-24 at Asilomar, California
Synchrony 2025 Roundtables
- Neuropsychiatric Deteriorations in Youth with ASD: Clinical Course Before and After Immunomodulation
Synchrony 2025 Industry Talks
SPEAKER: Douglas Gladstone, VP LinusBio
Imagine gaining access to autism insights years before symptoms appear, as early as one month of age, using nothing more than a single strand of hair. LinusBio’s Douglas Gladstone unveils their ClearStrand-ASD, a revolutionary non-invasive screening tool that decodes temporal molecular patterns in hair to reveal a first-of-its-kind autism biomarker. By empowering clinicians to spot developmental differences sooner, ClearStrand-ASD opens the door to timely interventions that research shows can boost IQ, language, and social skills during the brain’s most critical growth phases, long before the average U.S. diagnosis at age 4. Douglas showed us how this breakthrough can lift the cloud of uncertainty for families and why equitable access is vital to ensure no child with autism, regardless of background, is left behind.
SPEAKER: Blake Gurfein, Assistant Professor of Medicine (Adjunct) UCSF, Founder of Humanity Neurotech.
Autism spectrum disorder (ASD) has been linked to chronic neuroinflammation, oxidative stress, and mitochondrial and metabolic dysfunction in subsets of individuals. These abnormalities involve microglial and astrocyte activation, excess reactive oxygen species, impaired bioenergetics, and altered protein synthesis—factors that may contribute to ASD symptoms. Microtesla Magnetic Therapy (MMT) is a non-invasive intervention that delivers low-amplitude, radiofrequency magnetic fields to both cortical and deep brain structures and can be used at home. MMT targets inflammatory, oxidative, and mitochondrial pathways simultaneously. In human peripheral blood mononuclear cells, MMT suppressed NF-κB–dependent pro-inflammatory cytokine production. In rodent models of neuroinflammation, MMT reduced astrogliosis and microgliosis, preserved neural tissue, and attenuated neuronal oxidative stress. In THP-1 human monocytes, MMT enhanced protein synthesis under oligomycin A–induced mitochondrial inhibition, suggesting restored translational capacity during bioenergetic stress. A safety study in human subjects showed excellent treatment compliance and no adverse events. Building on these findings, Humanity Neurotech will conduct a randomized, double-blind, sham-controlled trial in individuals with ASD to assess safety, feasibility, behavioral outcomes, and neurometabolic effects via magnetic resonance spectroscopy. MMT’s ability to non-invasively modulate brain pathophysiology across multiple mechanistic domains, combined with its home-based delivery, positions it as a potential candidate for disease-modifying therapy in ASD.
This talk presented the results of an open-label trial of ethosuximide (ETS), a standard anti-seizure medicine, on 25 minimally-verbal, non-epileptic subjects with ASD (ages 4–23 years). The team measured word production and social function in subjects after 6 months of ETS, using the Vineland Adaptive Behavior Scale-3 (Vineland-3) and Caregiver’s Global Impression-Improvement scale, respectively. A new numerical language rating scale was also used at monthly clinic visits to rate subjects’ language progress. The influence of the subjects’ age and academic skills on language outcomes was also analyzed. Eighty percent of subjects (20/25) showed significant improvement in expressive language. Younger children (ages 4–8) exhibited greater language gains than teenagers and those with better academic skills advanced more than those with poorer school performance. Social interaction also improved. Subjects showed better eye contact, joint attention, and social engagement after 2–3 months of ETS treatment. ETS also affected the power spectra and coherence in EEGs of children with ASD. These findings suggest that ETS may offer a novel therapeutic approach for addressing core communication challenges in ASD.
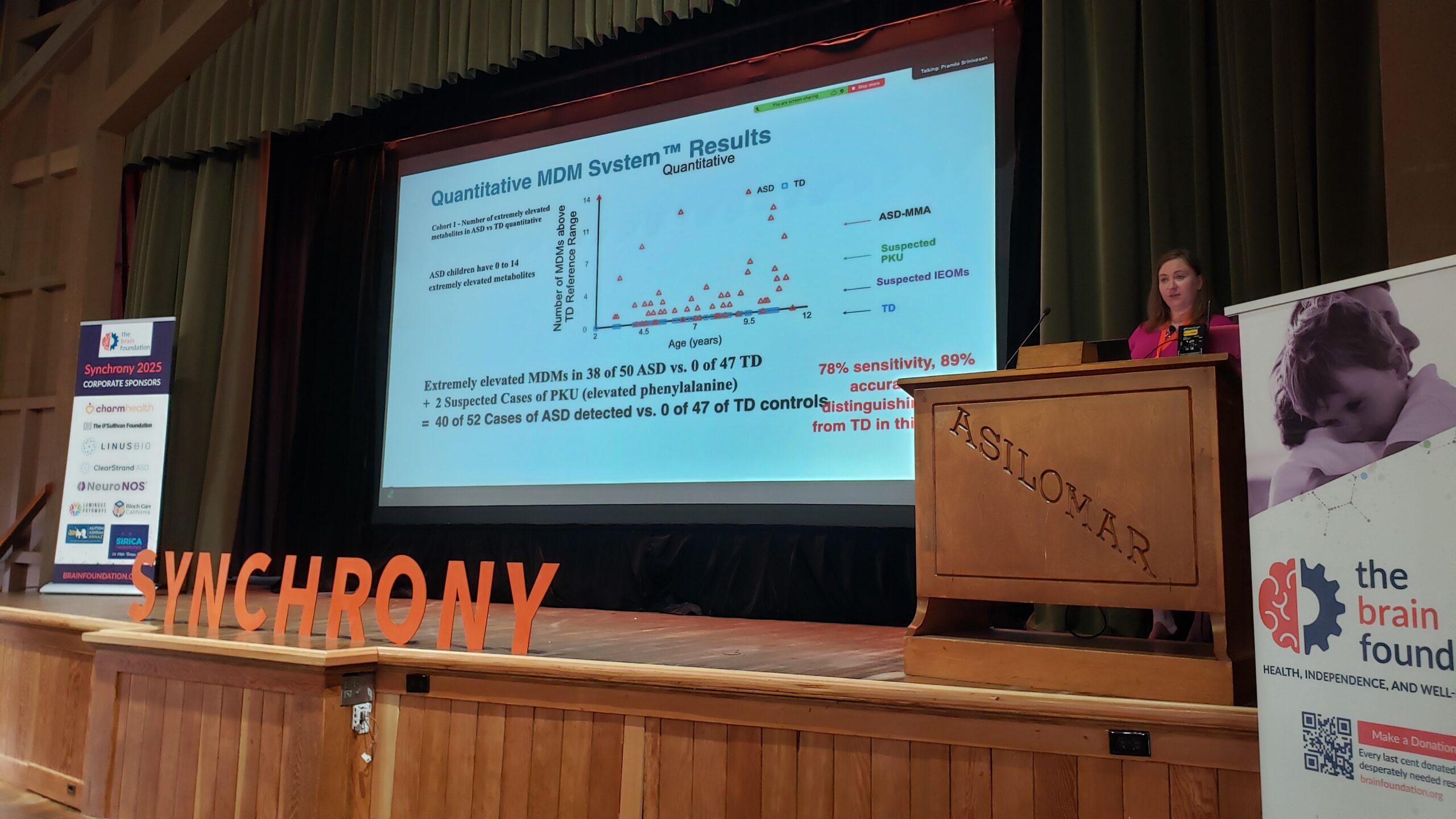
Many studies have demonstrated that children with autism have elevated levels of microbial metabolites that can affect brain and body function. For example, seventeen studies have all found that children with autism on average have higher levels of para-cresol. Para-cresol is produced by bacterial modification of phenylalanine or tyrosine. Para-cresol is known to adversely affect the gut, brain, neurotransmitters, mitochondria, kidneys, immune system, oxidative stress, and detoxification. Administration of para-cresol to mice causes autistic symptoms, and fecal transplant can reduce those symptoms. Urinary levels of para-cresol in children with autism correlates with severity of some symptoms. Microbiota Transplant Therapy was able to reduce levels of fecal para-cresol sulfate by 90% in children with autism. In her talk, Christina Flynn presented data on extremely elevated levels of para-cresol and other microbially-derived metabolites in children with autism compared to typically-developing children. She discussed the development of the Microbially-Derived Metabolite System (TM) for analysis of these metabolites and its utility for assessing gut health and serving as a diagnostic tool for autism.
SPEAKER: Katya Sverdlov, CEO of Jellikalite
In this talk Katya Sverdlov presented results of Jelikalite’s feasibility 2023 study, on which they based their current Pivotal RCT trial with Mount Sinai and SUNY Upstate Medical. The team conducted a small pilot RCT study (n=18) to test the usability, safety and efficacy of the re-designed Cognilum devices. Lessons Learned from the 2023 study include: 1. Size of the treatment effect depends on the severity of the initial symptoms, where the mid-point of the spectrum is the most responsive to the treatment. 2. The best respondents were those with initial CARS 36-50, size of the effect was 8 points reduction after treatment (4 point between group difference) as measured by CARS. 3. 8 minutes is the most effective dosage 4. The titration protocol is most effective, when starting at 2 minutes, and increasing incrementally by 2 minutes every week, until reaching 8-10 minutes. 5. The treatment may help to wean children off some medications (i.e. Respiridone) (case study 1) 6. Standardized autism tests are not sensitive to detect all types of improvements (case study 2). 7. Effectiveness of treatment for children older than 8 (case study) 3 The presentation will combine the average data together with case studies, to explicate the effectiveness of the treatment as it relates to specific symptoms.
PURPOSE: Autism spectrum disorder (ASD) is characterized by a lack of approved pharmacologic options that address its core neurodevelopmental symptoms. AST-001, a novel dopaminergic and GABAergic modulator developed by Astrogen, inc., is being advanced as a first-in-disease therapy targeting this unmet need. METHODS: A randomized, double-blind, placebo-controlled Phase 2 study (N=151; ages 2–11; KCT0007519) evaluated the efficacy and safety of AST-001 in children with ASD. The primary endpoint was change from baseline to Week 12 on the Korean Vineland-II Adaptive Behavior Composite (ABC). A 52-week open-label extension assessed durability and safety. Real-world data (RWD) from Pusan National University Yangsan Hospital (N=161) were analyzed to evaluate the natural progression of functional outcomes under standard-of-care treatments. RESULTS: AST-001 achieved statistically significant improvement in adaptive functioning at Week 12, representing the first global pediatric Phase 2 trial to reach significance on this stringent endpoint. Improvements were most pronounced in communication and motor domains, with a favorable safety and discontinuation profile. Long-term extension results demonstrated sustained benefit in 94% of responders. In contrast, RWD showed progressive decline in functional scores over two years with existing pharmacotherapies. Conclusion These data suggest AST-001 may serve as the first pharmacologic therapy targeting core ASD symptoms, particularly when administered during the neurodevelopmental “golden window” (ages 2–6). The program underscores Asia’s emerging leadership—especially Korea’s—in advancing global ASD drug development.
Synchrony 2025 research Presentations
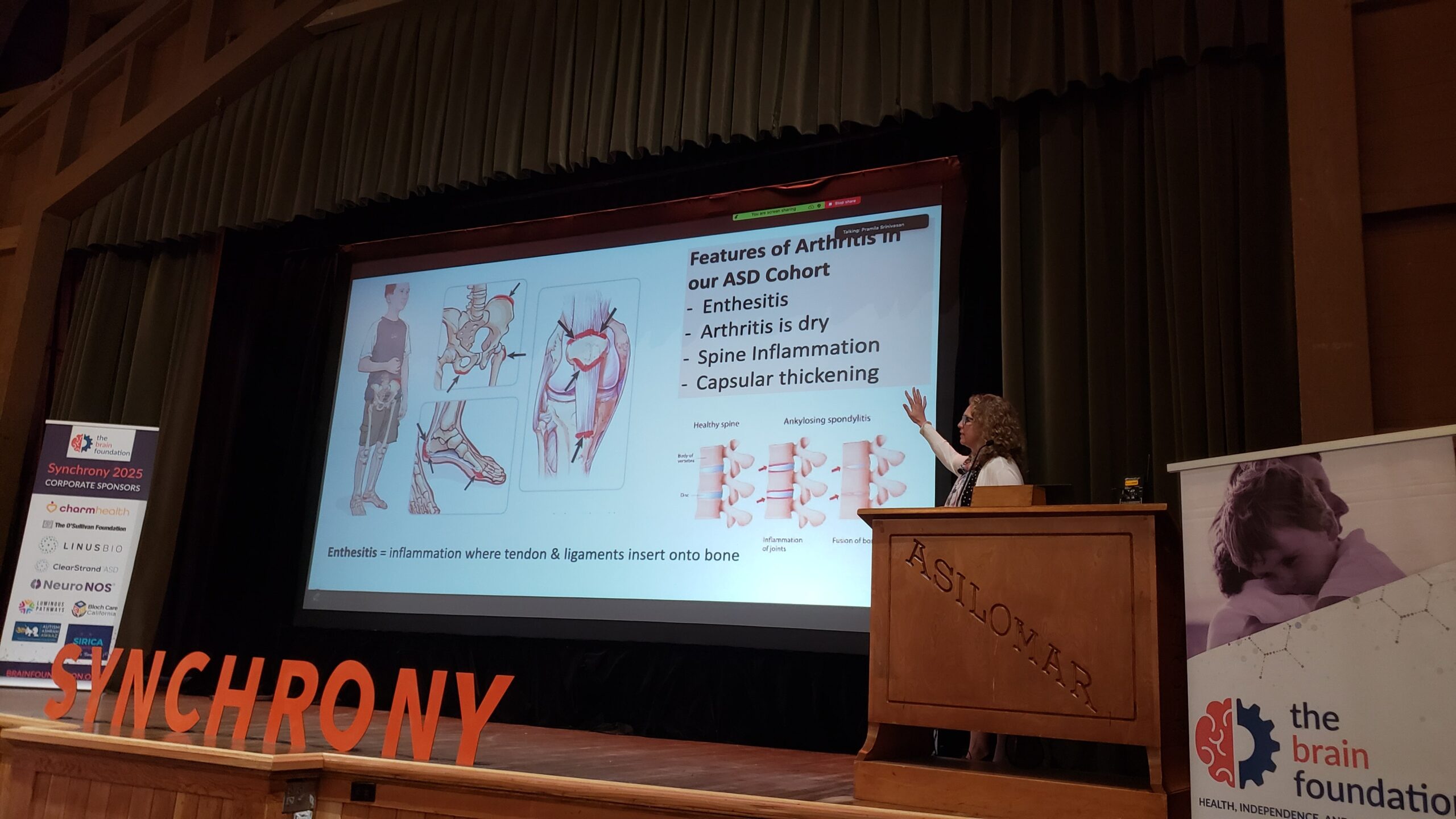
The causes of abrupt severe neuropsychiatric deteriorations among patients with previously stable Autism Spectrum Disorder (ASD) are poorly understood. These deteriorations – marked by profound loss of skills and escalated behavioral symptoms – present substantial challenges for care. Potential contributors include psychosocial stressors, metabolic stressors, infections, and immune-mediated processes. In this study, Dr Frankovich presents the relative incidences of autoimmune diagnoses (including arthritis), inflammatory markers, and other findings from 43 consecutive patients with ASD seen in the Stanford Immune Behavioral Health (IBH) clinic, a specialty program for children experiencing abrupt-onset neuropsychiatric deteriorations. While not all patients presented with a classifiable autoimmune disease at the time of deterioration, over half demonstrated markers of immune activation, with a third showing a marker of autoimmunity and /or nonspecific vasculopathy or vascular inflammation. Markers of anemia, diagnosis of arthritis, predominantly enthesitis-related arthritis and psoriatic arthritis were also present. No patients had rheumatoid arthritis. Arthritis was confirmed by imaging in 14/15 patients (93%). Among 10 patients who had joint imaging and did not meet established criteria for juvenile arthritis, 70% had evidence of joint effusions and/or synovitis, which may represent a forme fruste of arthritis. Additionally, 9/43 (21%) were found to have an autoimmune condition other than arthritis, including Celiac disease (n=3), psoriasis (n=2), autoimmune thyroiditis (n=2), inflammatory bowel disease (n=2), and SLE (n=1). Primary immunodeficiency was found in 3 patients (7%). These data underscore the importance of targeted immune evaluation—including inflammatory marker screening and musculoskeletal imaging—in ASD patients with abrupt behavioral regression.
Autism Spectrum Disorder (ASD) and Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) share immune-inflammatory underpinnings, with overlapping clinical symptoms. This retrospective, observational study evaluated the mid- and long-term outcomes of corticosteroid pulse therapy in 30 children and adolescents (age 3–18 years, mean 10.6) with ASD and PANS-related symptoms. Inclusion criteria comprised regressive ASD onset and/or immuno-allergic comorbidities, recurrent infections linked to ASD worsening, or at least one documented PANS episode. Patients were treated with pulse oral prednisone (90%) or intramuscular betamethasone (10%), with a variable rhythm (e.g. 5 days/months or 3 days every 21 every 21 days) tailored to symptom severity; 73% also received azithromycin. The Aberrant Behavior Checklist (ABC), the Children’s Yale-Brown Obsessive-Compulsive Scale (CY-BOCS), and the Yale Global Tic Severity Scale (YGTSS) were used as standardized outcome measures. At 12 months, significant improvements across multiple domains were observed such as reduced irritability, stereotypies, inappropriate speech, obsessive-compulsive symptoms, and tic severity. No significant adverse effects occurred. After 24 months, 60% of patients showed decreased ASD severity level, 30% remained stable, and 10% no longer met diagnostic criteria for ASD. No cases of clinical worsening were recorded. Nonetheless, emerging or persisting comorbidities, especially ADHD, highlighted a shift in developmental trajectories rather than a complete remission. These findings suggest corticosteroid therapy, alone or combined with azithromycin, may modulate ASD/PANS symptomatology. Controlled studies are warranted to confirm efficacy and clarify long-term trajectories.
Children with autoimmune encephalitis commonly have long term cognitive consequences even after effective treatment. We have established an animal model in mice of exposure to pathogenic NMDAR antibodies during a defined period of early postnatal development that leads to anatomic alterations in critical callosal circuits. We also show that these mice have permanent behavioral and electrophysiological consequences that reflect circuit dysfunction in callosal connectivity. This work establishes the potential that more generally autoimmune encephalitis in children may cause long-term disability by altering the fundamental properties of developing circuits.
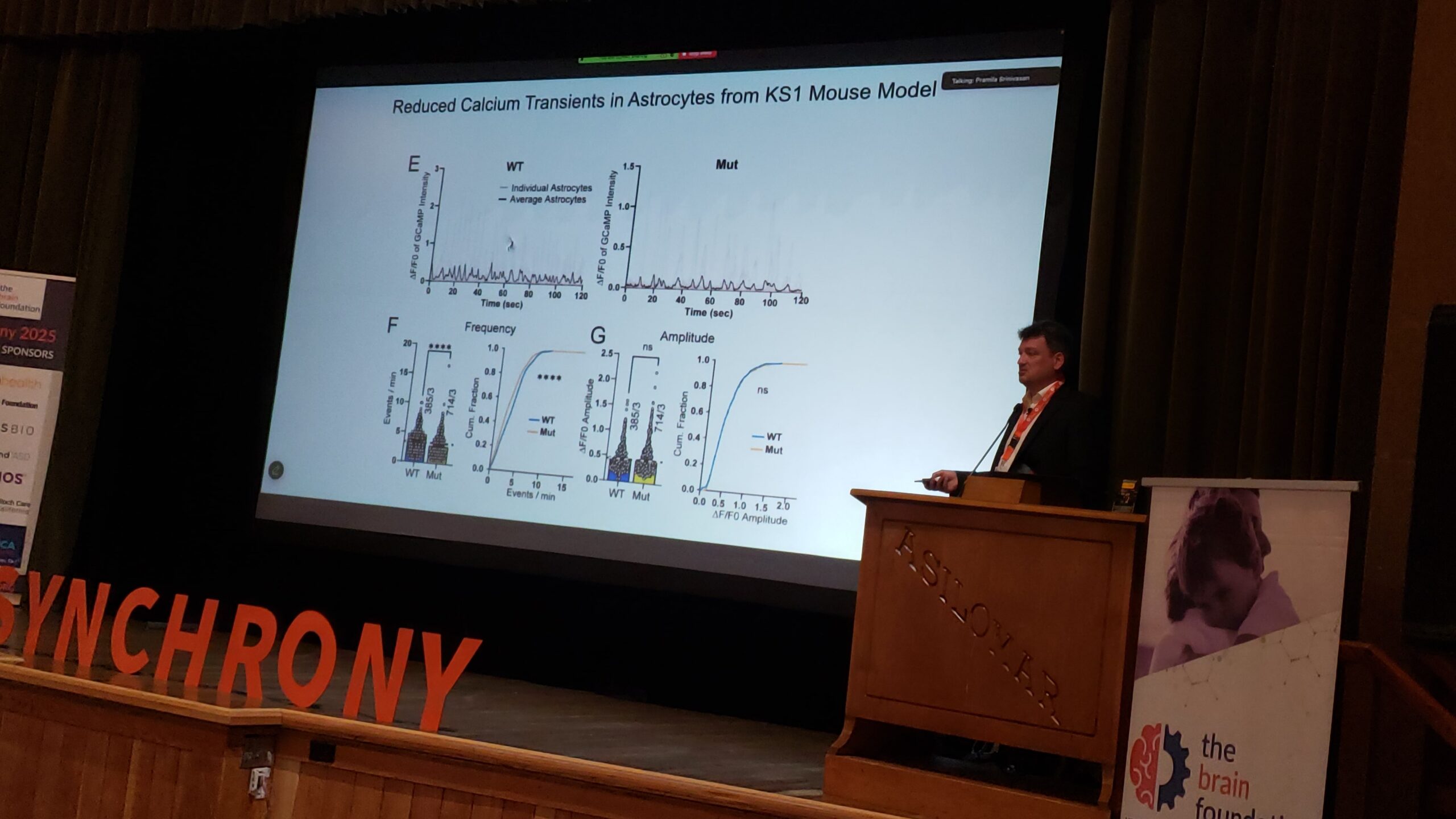
Brain development and function are highly reliant on adequate development and maintenance of vascular networks. As such, early cerebrovascular dysfunction can affect brain maturation by impacting trophic support and energy supply. Our recent published evidence in a 16p11.2 deletion mouse model of autism spectrum disorder (ASD) revealed neurovascular abnormalities associated with brain endothelial dysfunction postnatally, as well as a compensatory shift in adult brain metabolism. Yet, the endothelial alterations eliciting these changes remain unknown. To address this knowledge gap, we first isolated brain endothelial cells (ECs) from 14-day old 16p11.2-deficient male mice and wild-type littermates to assess endothelial parameters in vitro. We discovered that 16p11.2 deletion-induced endothelial dysfunction is linked to a bioenergetic failure with reduced intracellular ATP in ECs. Intra- or extra-cellular ATP supplementation rescued the function of 16p11.2-deficient ECs in vitro via P2 purinergic receptor activation, specifically P2Y2 receptors. Moreover, we find that 16p11.2- deficient ECs display distinct Ca 2+ responses following administration of extracellular ATP. Finally, engaging P2Y2 receptors with a selective agonist in vivo restored 16p11.2 deletion-associated mouse behaviors. Taken together, this study demonstrates that metabolic reprogramming of brain ECs via P2Y2 receptor activation may represent a new therapeutic avenue for ASD-associated cerebrovascular deficits.
When seeking treatment from either public or private healthcare providers, parents of children with ASD that exhibit evidence of chronic GI symptoms have historically encountered obstacles that impede their child from receiving appropriate and necessary care. Among the most significant issues in this setting are: (1) the inability of many healthcare providers to recognize GI symptoms in a non- or poorly communicative child and, (2) a misdiagnosis arising from a lack of relevant information (both molecular and histological) to accurately assess GI pathology and to effectively guide clinical decision-making in this unique patient population. The goal of this project is to provide an evidence-based framework that will produce a paradigm shift in the way chronic gastrointestinal symptoms in children with ASD are clinically evaluated. Existing data (e.g., Furlano et al. PMID: 11241044) show that significant molecular pathology is often lurking in the normal or near normal “unremarkable” light microscopic images of intestinal biopsy tissue of GI symptomatic ASD children. If results from the new comprehensive validation studies being performed in this project replicate the original findings, they would provide strong support for a change in prevailing clinical practice of the evaluation of GI symptomatic ASD children to include a more thorough evaluation of gastrointestinal biopsy tissue when the presenting symptoms are suggestive of an inflammatory phenotype.
Genetic variants in the TRIO gene increase risk for neurodevelopmental disorders (NDDs) including schizophrenia, autism, and related disorders. TRIO encodes a large protein with two guanine nucleotide exchange factor GEF domains for Rho family GTPases: GEF1 activates Rac1 and RhoG, and GEF2 activates RhoA. TRIO relays signals from cell surface receptors to coordinate cytoskeletal rearrangement and protein trafficking to regulate neuronal migration, axon and dendrite development, and synapse formation and function. We have investigated how heterozygosity for NDD-associated Trio variants – +/R1078Q (autism), +/K1431M (autism), +/K1918X (schizophrenia), and +/M2145T (bipolar disorder) – impacts mouse behavior, brain development, and synapse structure and function. Heterozygosity for different Trio variants impacts motor, social, and cognitive behaviors in distinct ways that model clinical phenotypes in humans. We have also found that a cluster of variants associated with intellectual disability and autism selectively impacts TRIO GEF1 activity, making it a potential target for therapy. We will discuss our ongoing strategy to target TRIO GEF1 activity therapeutically.
Neuroinflammation plays a central role in autism causation and several recent studies have reported activation of the NLRP3 inflammasome. Since oxidative stress promotes NLRP3 activity, we examined the relationship between antioxidant and methylation metabolic pathways and NLRP3 activation in THP-1 cell-derived macrophages. Time-dependent changes in metabolite levels were observed upon lipopolysaccharide (LPS) activation of NLPR3, monitored as IL-1 beta production. Multiple studies have documented low plasma cysteine levels in autism, so we examined NLRP3 activity under ASD-like cysteine-deficient growth conditions. A 20% reduction in cysteine caused a large deficit in the cellular level of reduced glutathione (GSH) and elevated levels of oxidized glutathione (GSSG), resulting in an 8-fold increase in GSH/GSSG, indicative of oxidative stress. Under this condition, LPS-induced NLRP3 activity was significantly increased, consistent with activation by oxidative stress. qPCR studies in postmortem ASD brain samples revealed up-regulation of inflammasome and cytokine gene expression in frontal cortex, along with increases in the antioxidant transcription factor Nrf2 and several of its target genes. Moreover, similar features were observed in brain tissue of BTBR mice, a model for both autism and type II diabetes. Our findings indicate an intimate relationship between inflammation and antioxidant status in autism.
We investigated the FDA-approved drug metformin for its potential to reverse behavioral and biochemical deficits in Gldc triplication mice, a schizophrenia mouse model. Metformin had a dose-dependent effect, with 300 mg/kg being more effective than lower doses. A single dose had no effect on working memory deficits, but 30-day chronic treatment reversed startle habituation and cognitive impairments while partially restoring social deficits. At molecular level, the expression of mitochondrial biogenesis related protein PGC1α, and neurotrophic factor BDNF expression was restored. We are investigating the role of metformin in modulating mitochondrial activity by assessing gene expression and activity dependent CREB activation. These analyses elucidate the mechanistic understanding of metformin on mitochondrial dynamics. Furthermore, we are developing strategies to identify neuronal ensemble activity patterns in the prefrontal cortex as potential biomarkers for evaluating metformin’s therapeutic efficacy. We are in the process of evaluating the role of metformin on other genetic mouse models (1q21.1 deletion and 15q13.3 deletion) of neurodevelopmental disorders. These studies will provide insights into the broader therapeutic potential of metformin in improving mitochondrial function across different genetic backgrounds. Establishing a common mechanistic link between mitochondrial dysfunction and neurodevelopmental disorders could enable development of targeted treatment strategies regardless of the underlying genetic mutation.
Human brain contains approximately 100 billion neurons, each forming thousands of synaptic connections (synapses), which serve as the smallest computational units in this complex network. Neurons, along with glial cells, work together in specialized networks, interacting at synapses in patterned ways that enable rapid, processed information transfer. Intellectual disability affects about 2–3% of the general population and is often caused by mutations that developmentally impair synapses, neural cells, and their circuits. Extensive research has identified and characterized mutations that contribute to or cause intellectual disability. Unfortunately, critical insights into the cellular and molecular mechanisms underlying these diseases are often lacking. A deeper understanding of the specific molecular makeup and signaling pathways involved in these conditions is essential. Human model systems in concert with mouse models are indispensable for advancing intellectual disability research.
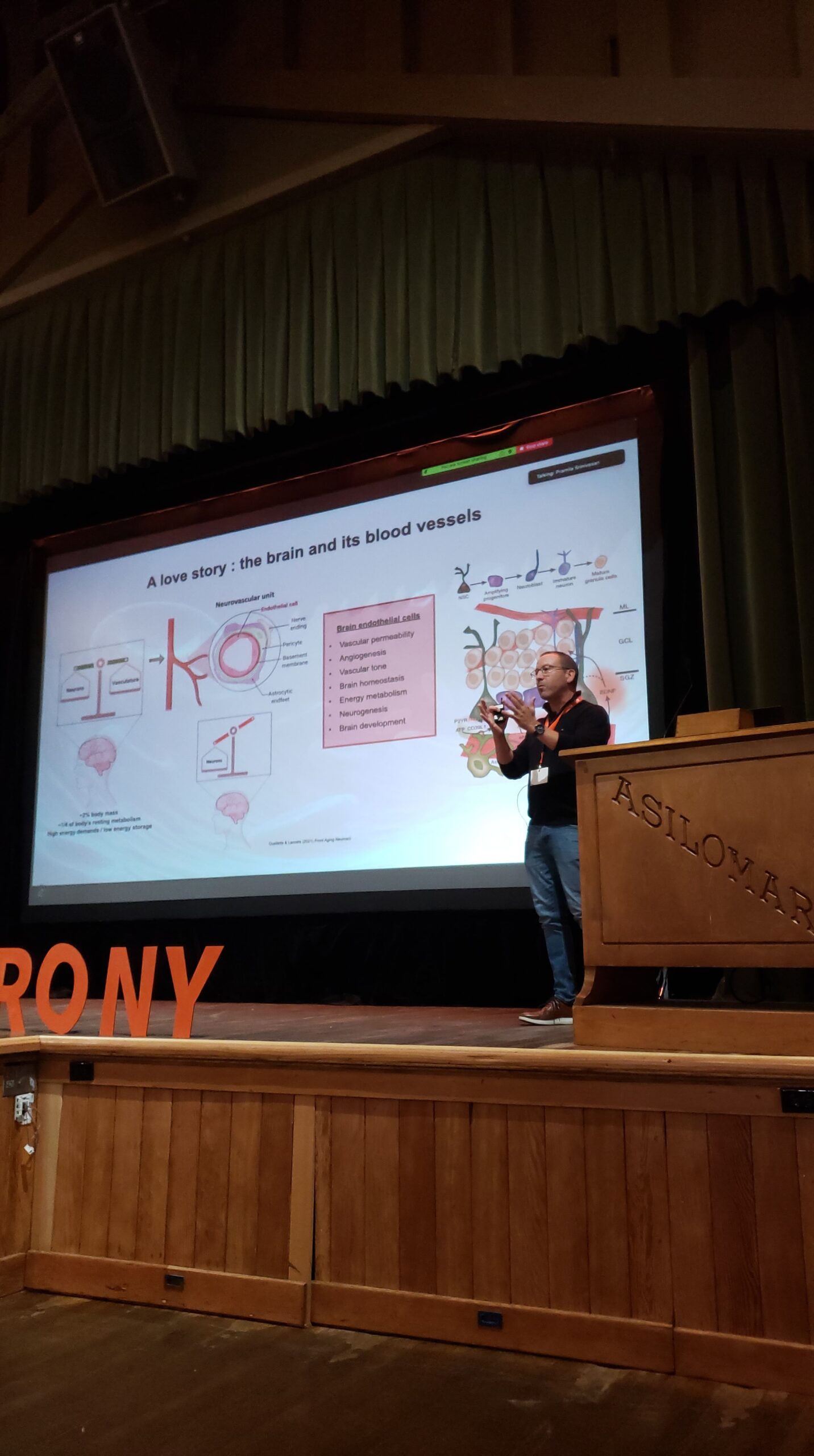
Diverse vascular cell subtypes tile the nascent vasculature in the prenatal human brain, following stage-specific developmental trajectories and distinct bioenergetic profiles. In addition, coordinated ensembles of endothelial and mural cells drive angiogenesis during prenatal human brain development. Finally, the blood brain barrier is one famous property of brain vascular cells. In general, this structure excludes cells and substances from the blood from entering the brain. During development, there may be selective openings in the blood brain barrier that stimulate brain development and could be harnessed therapeutically. In sum, the brain vasculature is emerging as an exciting topic in neuroscience and specifically for neurodevelopmental conditions.
Neurons communicate through neurochemical signals that either terminate at the postsynaptic process (“wired transmission”) or diffuse beyond the synaptic cleft to modulate the activity of larger neuronal networks (“volume transmission”). Molecules such as dopamine, serotonin, and neuropeptides such as oxytocin belong to the latter class of neurochemicals, called neuromodulators, and have been the pharmacological targets of antidepressants and antipsychotics for decades. However, until very recently, imaging the spatial and temporal propagation of neurochemical signals was not possible. To this end, we present a library of nanoscale near-infrared fluorescent nanosensors to image synaptic-scale neurochemical propagation of dopamine (Beyene et al. Science Advances 2019; Yang et al. Nature Protocols 2021), serotonin (Jeong et al. Science Advances 2019), and oxytocin (Mun et al. PNAS 2024), and describe how to implement our nanosensors to image neurochemical signaling in living brain tissue. We show that our oxytocin nanosensors can be used to study non-reproductive peer relationships in voles and find that oxytocin signaling is impaired in voles that show decreased peer relationship preferences. We finally discuss the relevance of our findings for supporting advances in understanding and treating autism spectrum disorders.
Autistic children with disproportionate megalencephaly (ASD-DM), defined by an enlarged brain relative to body height, exhibit higher rates of intellectual disability and more severe cognitive impairments compared to autistic children with typical brain sizes. However, the cellular and molecular mechanisms driving this neurophenotype remain poorly understood. To address this gap, we generated human induced pluripotent stem cells (iPSCs) from 40 typically developing (TD-N) and autistic children with and without disproportionate megalencephaly (ASD-N, ASD-DM). These individuals were assessed longitudinally from ages two to twelve years or beyond using MRI and comprehensive cognitive and medical evaluations. Using two dimensional and brain organoid models, we find that neural progenitor cells (NPCs) and cortical neural cells derived from ASD-DM individuals exhibit increased survival and suppressed cell death, despite heightened oxidative stress and ferrous iron accumulation. ASD-DM NPCs actively suppress apoptosis and ferroptosis through dysregulation of key proteins, including caspase-3 (CASP3) and glutathione peroxidase 4 (GPX4). Peripheral blood analyses further reveal elevated expression of GPX4 and other selenocysteine genes in ASD-DM children and their mothers, suggesting potential hereditary or environmental influences. Notably, GPX4 expression correlates with cognitive outcomes (IQ), identifying ferroptosis-related pathways as promising targets for early diagnosis and therapeutic intervention in ASD.
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental disorder influenced by both genetic and environmental factors. In this study, we examined the impact of gene-environment (G × E) interaction on ASD-like phenotypes using a preclinical rat model heterozygous for the metabotropic glutamate receptor 5 (mGluR5). Exposure to the environmental toxicant dibutyl phthalate (DBP) in these genetically susceptible animals resulted in elevated levels of peroxynitrite, as indicated by increased nitrotyrosine staining and upregulation of inducible nitric oxide synthase (iNOS). These molecular changes were associated with behavioral impairments, including reduced social interaction, increased repetitive behavior, and deficits in sensorimotor gating, which represent core features of ASD. Furthermore, significant impairments in social novelty preference and exaggerated marble burying behavior were observed exclusively in the mGluR5 heterozygous rats exposed to DBP, highlighting the specificity of the G × E interaction. Importantly, these changes were not observed in control groups, including wild-type littermates with or without DBP exposure and untreated or vehicle-treated mGluR5 heterozygous rats. This indicates a specific requirement for both genetic vulnerability and environmental insult to have the observed phenotypes. Notably, treatment with the peroxynitrite scavenger significantly reversed these behavioral abnormalities. These findings implicate peroxynitrite signaling and oxidative stress as central mechanisms underlying the effects of gene-environment interaction in ASD. Our results support the therapeutic potential of redox-modulating agents in mitigating ASD-related behavioral deficits.
Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) and Autism-Spectrum Disorder (ASD) share underlying immune dysregulation and suspected blood–brain barrier (BBB) involvement, yet the precise drivers of barrier disruption remain unclear. In this study, primary human brain endothelial cells, representing the physical BBB, were exposed to heat-inactivated plasma from 12 ASD individuals in active and improved states, PANS flare patients, and healthy controls. Barrier integrity was assessed using permeability assays, RNA sequencing, cytokine profiling, and microscopy. PANS flare plasma consistently induced BBB hyperpermeability, characterized by elevated IL-6 and MMP-9 levels, disruption of junctional complexes, extracellular matrix remodeling, and enhanced leukocyte adhesion. In contrast, ASD plasma showed heterogeneous effects, with approximately 42% of active cases producing marked barrier disruption that improved upon symptom resolution. Although circulating MMP-9 levels were elevated in both PANS and ASD, they did not reliably predict BBB dysfunction in ASD. Notably, both MMP-9 inhibition and activation of the α7 nicotinic acetylcholine receptor restored barrier integrity and junctional architecture. These findings suggest that BBB disruption is a hallmark of PANS and occurs in a subset of ASD cases, with MMP-9–mediated mechanisms playing a partial role, and that therapeutic strategies targeting MMP-9 and α7-nAChR pathways may benefit pediatric neuroimmune disorders.
Computational psychiatry studies suggest that individuals with autism spectrum disorder (ASD) inflexibly update their expectations. Here we leveraged high-yield rodent psychophysics, extensive behavioral modeling and brain-wide single-cell extracellular recordings to assess whether mice with different genetic perturbations associated with ASD show this same computational anomaly, and if so, what neurophysiological features are shared across genotypes. Mice harboring mutations in Fmr1, Cntnap2 or Shank3B show a blunted update of priors during decision-making. Compared with mice that flexibly updated their priors, inflexible updating of priors was associated with a shift in the weighting of prior encoding from sensory to frontal cortices. Furthermore, frontal areas in mouse models of ASD showed more units encoding deviations from the animals’ long-run prior, and sensory responses did not differentiate between expected and unexpected observations. These findings suggest that distinct genetic instantiations of ASD may yield common neurophysiological and behavioral phenotypes. These deficit parallel human observations, opening an exciting translational opportunity.
Hoxb8 mutant mice show compulsive behavior similar to the repetitive behaviors in the Autism spectrum disorder in comorbidity with trichotillomania, a human obsessive-compulsive-spectrum disorder. The only Hoxb8 lineage-labeled cells in the brains of mice are microglia, suggesting that defective Hoxb8 microglia caused the disorder. We have shown the presence of two microglia subpopulations: canonical, non-Hoxb8 microglia and Hoxb8 microglia. Unlike non-Hoxb8 microglia, Hoxb8 microglia progenitors appear to be generated during the second wave of yolk sac hematopoiesis, then detected in the aorto-gonad-mesonephros (AGM) and fetal liver, where they are greatly expanded, prior to infiltrating the E12.5 brain. In this work we have investigated the effect of sensory deprivation on Hoxb8 microglia function. Using whisker trimming based sensory deprivation model, our result suggests that the sensory deprivation during the critical period reduces Hoxb8 but not non-Hoxb8 microglia density in contralateral hemisphere to the deprived hemisphere without affecting the non-Hoxb8 microglia population. The effect is enhanced significantly in Hoxb8 mutants. Given the crucial role of Hoxb8 microglia in synaptic pruning, our current data suggests that Hoxb8 microglia are highly sensitive to synapse density during critical period. Furthermore, we demonstrate the unique capabilities of Hoxb8 microglia in synaptic pruning compared to non-Hoxb8 microglia. Non-Hoxb8 microglia significantly outnumber Hoxb8 microglia, but they cannot compensate for the loss of Hoxb8 function in Hoxb8 microglia, suggesting further crucial differences between the two subpopulations during critical period, synaptic development, synaptic pruning and postnatal synaptic maturation.
SYNCHRONY SPONSORS






roundtable
discussions
clinical
pearls
researchers’
‘mini’ course
one-on-one
mentoring
research
presentations
industry
updates